Abstract
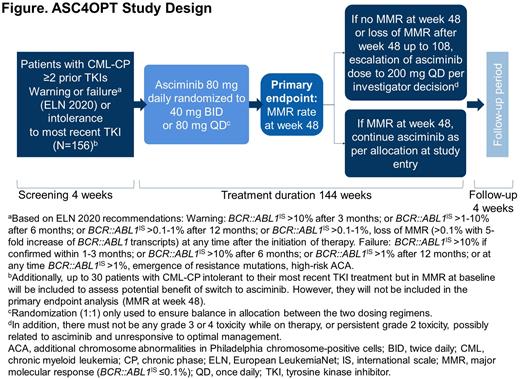
Introduction Tyrosine kinase inhibitors (TKIs) are the standard of care in the treatment of chronic myeloid leukemia (CML). However, patients (pts) may experience resistance or intolerance to successive lines of TKI therapy. Asciminib is the first BCR::ABL1 inhibitor that works by Specifically Targeting the ABL Myristoyl Pocket (STAMP). It is approved in the United States for the treatment of adults with Philadelphia chromosome-positive (Ph+) CML in chronic phase (CP) previously treated with ≥2 TKIs (at 40 mg twice daily [BID] or 80 mg once daily [QD]), and also Ph+ CML-CP with the T315I mutation (at 200 mg BID). This was based on the Phase 3 ASCEMBL trial (Rea et al. Blood 2021) and a Phase 1 study (Cortes et al. ASH 2020). The ASC4OPT (NCT04948333) trial was initiated to investigate the potential optimization of treatment with asciminib in adult pts with CML-CP previously treated with ≥2 TKIs (≥3rd-line). ASC4OPT will explore 3 aspects of treatment optimization: 1. Pt population, ASCEMBL inclusion criteria were based on the European LeukemiaNet (ELN) treatment recommendations from 2013. ASC4OPT will re-evaluate asciminib in an expanded ≥3rd-line population using the ELN 2020 treatment recommendations for warning and failure criteria. 2. Simplified posology, asciminib dosing in ASCEMBL was 40 mg BID. BID dosing may affect optimal compliance for pts and ASC4OPT will investigate both asciminib 40 mg BID and 80 mg QD. 3. Dose escalation, with limited treatment options in the ≥3rd-line setting, ASC4OPT will explore whether pts not in major molecular response (MMR) at ≥48 weeks (wks) could benefit from a potential dose escalation to 200 mg QD.
Methods ASC4OPT is an international, multi-center, non-comparative, Phase 3b study in pts aged ≥18 years with CML-CP previously treated with ≥2 TKIs. The trial will enroll approximately 156 pts who are in treatment failure or warning (ELN 2020) or intolerant to the last prior TKI and not in MMR at baseline. Up to 30 additional pts in MMR at baseline and intolerant to their most recent TKI treatment will also be enrolled; these pts will not be included in the primary endpoint analysis, however all assessments will be performed. Pts are excluded if they have had previous or planned hematopoietic stem cell transplantation, second CP after previous progression to accelerated phase/blast crisis, or known presence of the BCR::ABL1 T315I mutation at any time prior to study entry. Pts will be randomized 1:1 to receive asciminib 40 mg BID or 80 mg QD. In pts not achieving MMR at 48 wks or losing response after wk 48 and up to wk 108, asciminib dose may be escalated to 200 mg QD if in the investigator's opinion the pt may benefit from the escalation. In addition, for dose escalation there must not be any grade 3 or 4 toxicity while on therapy, or persistent grade 2 toxicity, possibly related to asciminib and unresponsive to optimal management (Figure). The primary endpoint is MMR rate (BCR::ABL1IS ≤0.1%) at wk 48 in pts not in MMR at baseline. Secondary endpoints include MMR rate at wks 12, 24, 36, 72, 96 and 144 in pts not in MMR at baseline, MMR rate at wk 48 for pts in MMR at baseline, time to MMR, rate of early responses of BCR::ABL1IS ≤10% and ≤1% at wks 12, 24, 36 and 48, deep molecular responses (rates of MR4.0 [BCR::ABL1IS ≤0.01%] and MR4.5 [BCR::ABL1IS ≤0.0032%] at wks 12, 24, 36, 48, 72, 96 and 144), rate of cytogenetic response at wk 48 and at end of treatment (up to 144 wks), and patient-reported outcomes and quality of life.
Current status As of July 2022, 77 patients have been enrolled in the study.
This study is sponsored by Novartis Pharmaceuticals.
Disclosures
Breccia:Novartis, Incyte, Pfizer, BMS, Abbvie: Honoraria. Turkina:Pfizer: Other: Travel, Accommodation, Expenses , Speakers Bureau; Fusion Pharma: Speakers Bureau; Novartis: Other: Travel, Accommodation, Expenses , Speakers Bureau. Boquimpani:Novartis, Jansen, Pint Pharma, EMS: Honoraria. Chuah:Pfizer: Other: Travel, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Steward Cross: Korea Otsuka International Asia Arab: Honoraria; Otsuka [Philippines] Pharmaceutical: Honoraria; Korea Otsuka Pharmaceutical: Honoraria; Novartis: Honoraria. Sharf:Boehringer Ingelheim: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees. Di Caprio:Novartis: Current Employment. Hoch:Novartis: Current Employment, Current equity holder in private company. Yssel:Novartis: Current Employment, Current equity holder in private company. Zhang:Novartis: Current Employment. Hochhaus:Pfizer: Research Funding; Novartis: Research Funding; Bristol Myers Squibb: Research Funding; Incyte: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal